| Title | Hypercalcaemia: diagnosis and treatment options in the dog and cat | |
| Author | Joao Felipe de Brito Galvao MV, Dennis Chew DVM Dipl. ACVIM, Patricia Schenck DVM PhD | |
| Status of this article | 5 Removed | |
| Date | 03/07/2017 | |
| Jobs related | ||
| Small animal related | ||
| Large animal related | ||
| Equine related | ||
| Exotics related | ||
| Vetboss Employment related | ||
| Vetboss Business related | ||
| Vetboss Finance related | ||
| Vetboss Small animal related | ||
| Vetboss Large animal related | ||
| Vetboss Equine related | ||
| Vetboss Exotics related | ||
| Content | Hypercalcaemia: diagnosis and treatment options in the dog and cat
Joao Felipe de Brito Galvao MV and Dennis Chew DVM Dipl. ACVIM, The Ohio State University, Columbus, OH, USA Patricia Schenck DVM PhD, Michigan State University, Lansing, MI, USA
Introduction Calcium is required for a number of intra and extra-cellular functions, as well as for skeletal support. Total calcium (tCa) is the form of calcium most commonly analysed. Circulating calcium exists in three fractions: ionised, complexed (bound to phosphate, bicarbonate, etc.), and protein-bound (1). In normal dogs, ionised, complexed and protein-bound calcium account for 55%, 10%, and 35% of serum total calcium respectively (2). In cats, values are similar (3). The ionised calcium (iCa) fraction is the biologically active fraction that is regulated and so is the gold standard for evaluation of calcium status. Regulation of serum calcium is complex and involves the integrated actions of parathyroid hormone (PTH), vitamin D metabolites, and calcitonin (4). PTH is involved in the fine regulation of blood calcium concentration. When ionised calcium concentration decreases, PTH production is stimulated within the main cells of the parathyroid gland. PTH increases blood calcium concentration by a variety of effects; it also causes phosphaturia, which decreases serum phosphorus. Calcitonin is a minor player in calcium metabolism, serving mainly to limit the degree of hypercalcaemia following a calcium-rich meal. In hypercalcaemia, the interaction of calcium and phosphorus is important. Whenever the product of the tCa (mg/dL) multiplied by the phosphorus concentration (mg/dL) exceeds ~70, tissue mineralisation is likely. This is critical since the soft tissues that most often mineralise are found in the kidneys, stomach and vascular system.
Diagnostic tests of calcium metabolism 1) Serum total calcium tCa is part of most routine serum profile evaluations. It is usually measured using a colourimetric method, and may be spuriously elevated if hyperlipaemia or haemolysis is present. Although ionised calcium is the biologically active fraction, many clinicians rely on serum tCa concentrations to predict iCa status. Adjustment formulas to correct tCa for the albumin or total protein concentration are not recommended in dogs or cats; various studies have shown that these can be poorly correlated and even lead to misdiagnosis (5,6). 2) Serum ionized calcium iCa is a better indicator of disease status and can be measured in serum or heparinised plasma. Note EDTA plasma is unacceptable as it can give a falsely decreased result. For the most accurate measurement samples should be collected and handled anaerobically because mixing serum and air can decrease the iCa level (7). Some laboratories have developed species-specific pH adjustment formulas to allow measurement of aerobically handled samples. 3) Parathyroid hormone An intact PTH assay provides accurate determination of PTH concentration. Serum or separated plasma samples can be used but should be kept refrigerated or frozen prior to assay. PTH should be evaluated at the same time as iCa. 4) Parathyroid hormone related protein PTHrP is a hormone that is secreted by some malignant neoplasms; it can bind to PTH receptors in the kidneys and bone and result in humoral hypercalcaemia of malignancy (HHM). PTHrP is sensitive to degradation, and appears to be more stable in separated EDTA plasma (unpublished observation) as compared to serum. Separated plasma should be kept frozen prior to PTHrP measurement. 5) Vitamin D metabolites Metabolites of vitamin D are chemically identical in all species. Concentration of serum 25-hydroxyvitamin D is a good indicator of vitamin D ingestion. Serum or separated plasma can be assayed, but samples should be protected from light to inhibit degradation. Calcitriol (1,25-dihydroxyvitamin D) is the active vitamin D metabolite, but unfortunately laboratory analysis is not widely available.
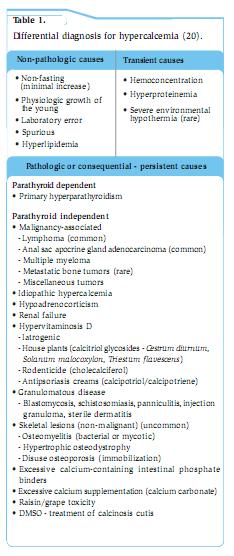
Clinical signs Anorexia, PU/PD, lethargy, and weakness are the most common clinical signs in dogs with hypercalcaemia, but individual animals often display remarkable differences in clinical signs despite similar magnitudes of hypercalcaemia. Though PU/PD are frequently described as early findings, this is far less common in cats as they appear to maintain an ability to concentrate urine to a far greater degree. Simultaneous disturbances in other electrolyte concentrations, as well as organ dysfunction secondary to hypercalcaemia, all contribute to clinical signs, laboratory abnormalities, and lesions. The severity of clinical signs and development of lesions of hypercalcaemia depend not only on the magnitude of hypercalcaemia but also on its rate of development and duration. For instance, dogs with the same level of hypercalcaemia can present with very different clinical signs. A dog with primary hyperparathyroidism may have only PU/PD, while a dog with lymphoma or anal sac adenocarcinoma may present with vomiting, anorexia, and PU/PD despite the same level of hypercalcaemia. Clinical signs of hypercalcaemia in cats vary from absent to severe but are usually insidious and often not noticed by owners. The clinical signs differ among patients and may be referable to one or more body systems. Signs may be nonspecific (e.g. lethargy, anorexia) or referable to the urinary system (e.g. PU/PD, dehydration, hematuria/pollakiuria/dysuria associated with urolithiasis), the gastrointestinal system (e.g. vomiting, constipation), neuromuscular system (e.g. seizures, weakness) or cardiac system (arrhythmias). Cats with primary hyperparathyroidism can have palpable cystic parathyroid nodules. Differential diagnosis, work-up and treatment Table 1 provides a list of causes of hypercalcemia. Characterisation of the hypercalcaemia as transient or persistent, pathologic or nonpathologic, mild or severe, progressive or static, and acute or chronic is helpful in determining its cause. Persistent, pathologic hypercalcaemia occurs most often in association with malignancy, especially in the dog (>50% of cases). Previously it was thought that CKD followed by malignancy was the most common cause of hypercalcaemia in cats (8). Recently, it appears that the number one cause of ionised hypercalcaemia is idiopathic, followed by CKD, and then malignancy (9). Other sporadic causes of hypercalcaemia include hypoadrenocorticism, primary hyperparathyroidism, hypervitaminosis D and inflammatory disorders. Parathyroid-independent hypercalcaemia is more common in cats than dogs. It is often difficult to determine the cause of hypercalcaemia in animals with mild or transient hypercalcaemia. It is important to ensure that the hypercalcaemia initially detected is repeatable (Table 1 and Figure 1). The first step is to determine whether the hypercalcaemia is parathyroid dependent (parathyroid gland disease causing hypercalcaemia) or parathyroid independent (normal parathyroid glands suppressing PTH secretion due to hypercalcaemia). Ultrasonography of the cervical region may help; however, the absence of an enlarged parathyroid does not rule out parathyroid dependent hypercalcaemia, emphasizing the importance of measuring PTH. In parathyroid-independent hypercalcaemia, parathyroid glands are not enlarged or may not be identified; some may be atrophic if hypercalcaemia from malignancy or hypervitaminosis D has been long standing. PTHrP evaluation may help if malignancy is suspected, but in itself is not diagnostic of malignancy. Measurement of 25-hydroxyvitamin D is useful in cases of potential excessive cholecalciferol or ergocalciferol ingestion and measurement of 1,25 dihydroxyvitamin D (calcitriol) is useful if excess calcitriol is the cause of hypercalcaemia (but this is rare). Patients with cytopoenias should undergo bone marrow evaluation if the diagnosis has not been established. Bone survey of all bones is sometimes useful in finding lesions even in those without demonstrable bone pain (e.g. from multiple myeloma). The approach to treatment depends on the severity of clinical signs, the underlying cause, and the magnitude of hypercalcaemia. The decision-making process will be guided by matters such as how quickly hypercalcaemia develops, if the calcium level continues to increase, the presence of hyperphosphataemia, the presence of severe acid-base disturbances, the level of renal function, and the status of brain function. Rapidly rising levels of hypercalcaemia justify more aggressive intervention and are most characteristic of malignancy or hyper-vitaminosis D. There is no single treatment protocol consistently effective for all causes of hypercalcaemia (Table 2). **Note: furosemide should never be given to a dehydrated patient or before IV fluids** Glucocorticosteroids provide a second tier of treatment for cases that do not respond adequately to tier one treatment. They can reduce the magnitude of persistent hypercalcaemia in patients with lymphoma (cytolysis), multiple myeloma, hypoadrenocorticism, hypervitaminosis D, or granulomatous disease, but they have minimal effect on other causes of hypercalcaemia and steroids should be withheld if a definitive diagnosis has not been established. Chronic steroid administration may be needed to control hypercalcaemia in feline idiopathic hypercalcaemia where dietary alterations are not effective. The third tier is to add a bisphosphonate for the control of hypercalcaemia. These drugs act by reducing the number and action of osteoclasts. They can be considered for treatment of chronic idiopathic hypercalcaemia of cats that has not responded to dietary therapy and following rehydration in cases of severe hypercalcaemia, especially when associated with hypervitaminosis D and malignancy where surgery or chemotherapy is ineffective. The authors have used bisphosphonates to decrease circulating calcium in inoperable cases of anal sac adenocarcinoma. Malignancy-associated hypercalcaemia The most common cause of hypercalcaemia in dogs is associated with neoplasia and may be due to HHM or LOH - local osteolytic hypercalcaemia due to local osteolytic mechanisms. LOH includes that which develops following solid tumours metastasising to bone, and haematologic malignancies in bone marrow due to local production of bone-resorbing factors (most commonly in multiple myeloma and lymphoma). HHM malignancies commonly seen in dogs include T-cell lymphoma (often mediastinal (Figure 2)) and apocrine anal sac adenocarcinomas (Figure 3). Clinical signs from the latter may be linked to hypercalcaemia (PD/PU, anorexia, and weakness), a mass in the perineum (tenesmus, ribbon-like stools, increased odour, and protruding mass), a mass in the sublumbar region, or more distant metastases. HHM also occurs in dogs with thymoma, myeloma, melanoma, or carcinomas originating in the lungs, pancreas, thyroid gland, skin, mammary gland, nasal cavity, clitoris and adrenal medulla. PTHrP levels are highest in dogs with apocrine adenocarcinomas of the anal sac and carcinomas associated with HHM. Lymphoma and squamous cell carcinoma are the two most common malignancies to cause hypercalcaemia in cats (8). Note primary bone tumours are often not associated with hypercalcaemia in dogs or cats. Chronic kidney disease (CKD) Hypercalcaemia occurs sporadically in dogs and cats with CKD. Many animals with CKD have normal tCa concentrations. Note hypercalcaemia can cause renal failure or develop as a consequence of CKD. In CKD, elevated tCa with normal iCa occurs in 11% of dogs (6) and from 11.5% (10) to 58% (11) of cats depending on the population studied. The incidence of elevated tCa increases with severity of azotaemia, and (in dogs) is usually due to an increase in the complexed calcium fraction (12). Deleterious effects of hypercalcaemia occur only if there is increased iCa concentration - emphasizing the need to measure iCa levels in CKD patients. Idiopathic hypercalcaemia in cats (IHC) Within the past 20 years, IHC has been recognized in cats as one of the most common causes of hypercalcaemia. Most cats (perhaps 50%) have no clinical signs, although mild weight loss, inflammatory bowel disease, chronic constipation, vomiting, and anorexia can all be noted. Uroliths (often oxalates) are observed in about 15% of cases. Age range can be from 0.5 - 20 years, and long-haired cats are over-represented. Typically, serum iCa is increased, PTH is normal, PTHrP is negative, and both serum iMg and 25-hydroxy-vitamin D concentrations are normal. Serum calcitriol is usually in the reference range or suppressed. Specific treatment for IHC is impossible because the pathogenesis remains unknown. Increased dietary fibre decreases serum calcium in some cats. Higher fibre diets may decrease intestinal absorption of calcium due to decreased transit time, but the effects of fibre are complex and depend on the types and amounts of fibre present. These “high fibre diets” are usually supplemented with extra calcium, and therefore calcium content does not explain why these diets are sometimes successful in treating IHC. Alternatively feeding a renal-type diet may result in normocalcaemia in some IHC cats; these diets are generally low in calcium and phosphorus and are considered alkalinising or at least less acidifying than maintenance diets. Acidifying diets increase the availability of the ionised form of calcium facilitating intestinal absorption; they also increase bone resorption which can contribute to hypercalcaemia. Some cats that show an initial decrease in serum calcium concentration following any type of dietary change will have a return to hypercalcaemia over time. For cats that do not respond to a change in diet, prednisolone therapy (5-20mg/cat/day) may help, although hypercalcaemia may later return despite maximal doses of prednisolone. Finally oral bisphosphonate preparations such as alendronate can be considered. A pilot study on the use of alendronate by the authors shows promise for long-term treatment of IHC (13) and is their first choice to treat this condition. Typically cats are dosed at 10 mg/week, although some cats require up to 20 mg/week (Table 2). ICa levels should be checked at 2-3 weeks, then 1 month, 2-3 months, and then every 4-6 months as long as the iCa is within normal limits. Given the associated risk of oesophagitis and stricture in humans, it is recommended to “butter” the cat’s lips and follow the dose with a syringe of water to prevent the pills from getting caught in the oesophagus; note oesophagitis following alendronate treatment has not been observed in cats. An overnight fast before dosing, with feeding 2h later, is recommended to maximize absorption. Hypoadrenocorticism This is the 2nd most common cause of hypercalcaemia in dogs based on evaluation of tCa but was seen in only 5% of dogs diagnosed with ionized hypercalcaemia (14). It is rarely recognized in cats. A correlation between the degree of hyperkalaemia and hypercalcaemia in dogs is detected when the serum potassium concentration is >6.0-6.5 mEq/L. Typical hypoadrenocorticism dogs are more likely to be acidotic, azotaemic and hypercalcaemic than atypical hypoadrenocorticism cases (15). Serum tCa concentration rapidly returns to normal after 1-2 days of corticosteroid therapy, and IV volume expansion can return serum calcium concentration to normal within a few hours. Hypoadrenocorticism should always be included in the differential diagnosis of hypercalcaemia because clinical signs of hypoadrenocorticism and hypercalcaemia can be similar. Hypervitaminosis D Hypervitaminosis D from consumption of cholecalciferol-containing rodenticides is often associated with the development of hypercalcaemia within 24h of ingestion. The magnitude of hypercalcaemia is often severe and mild to moderate hyperphosphataemia is often noted; soft tissue mineralization is usually more severe in these cases for this reason. Azotaemia is initially absent but can develop, usually within 72h, as a result of renal lesions caused by hypercalcaemia. Topical ointments containing calcipotriene for treatment of human psoriasis can result in hypercalcaemia when toxic quantities are ingested by dogs or cats. Phosphorus, tCa, and iCa are elevated with calcipotriene toxicity, and most develop acute renal failure. Hypercalcaemia decreases after several days rather than being prolonged for weeks to months. Ingestion of toxic plants that contain glycosides of calcitriol is a potential cause of hypercalcaemia. Measurement of serum 25-hydroxyvitamin D concentration can provide evidence for hypervitaminosis D after exposure to cholecalciferol/ergocalciferol but is not helpful when hypercalcaemia is created by other vitamin D metabolites. Primary hyperparathyroidism This is an uncommon cause of hypercalcaemia in dogs and is even rarer in cats. Excessive and inappropriate secretion of PTH by the parathyroid glands characterises this condition; hypophosphataemia is also common. Most cases are due to a single parathyroid adenoma (16). However, recent reports (17) suggest hyperplasia is a frequent occurrence and it can be difficult to distinguish between hyperplasia and adenoma. Neck ultrasound localises the affected parathyroid gland. Non-visible glands (may be atrophied) or glands with a widest measurement <2 mm are considered normal. Glands measuring 2-4 mm are considered borderline abnormal, suggestive of hyperplasia (especially if more than one gland is affected) and glands >4 mm are characteristic of an adenoma (18). Clinical signs related to hypercalcaemia are either mild (PU/PD, lethargy, weakness), or absent in many affected dogs. Calcium-containing uroliths (which may be palpable) and urinary tract infections occur in about 30% of patients with primary hyperparathyroidism (16). Serum iCa concentration is elevated and PTH is inappropriately high for the level of hypercalcaemia (iCa should cause PTH level to be in the lower half of the reference range). In one study, PTH was within reference limits in 73% of cases (16). It has been suggested that dogs with multiglandular disease should have 3 or 3.5 glands removed. However, it is possible that clinical signs will not ameliorate until removal of all glands, in which case dogs may become hypocalcaemic and hypothyroid requiring life-long calcitriol and thyroxin supplementation. The frequency of parathyroid hyperplasia has become increasingly common (authors’ unpublished observations). Additionally, some dogs can have unidentifiable hyperplastic ectopic parathyroid tissue, which may require medical management (e.g. alendronate) due to inoperability. It has been recommended to pre-treat dogs (19) with severe pre-operative hypercalcaemia (tCa >18 mg/dL) with calcitriol for 3-5 days (Table 2) and whilst this may transiently worsen hypercalcaemia, it can lessen the degree of severe post-operative hypocalcaemia, which requires intense ICU care. It has been also suggested to use bisphosphonates in severely hypercalcaemic patients due to “hungry bone syndrome” to prevent post-op hypocalcaemia despite the use of calcitriol; most cases of hypocalcaemia occur 2-6 days (12h to 20 days) after surgery (19).
Conclusion Appropriate treatment and prognosis will depend on the cause of the hypercalcaemia and it is therefore essential to search for the cause of persistent hypercalcaemia using an organised approach to correctly diagnose the underlying cause (Figure 1). Species, signalment, history and clinical signs play an important role in prioritising the differential diagnosis list.
This article was kindly provided by Royal Canin, makers of a range of veterinary diets for dogs and cats. For the full range please visit www.RoyalCanin.co.uk or speak to your Veterinary Business Manager:
References 1. Schenck PA, Chew, D.J., Nagode, et al. Disorders of calcium: Hypercalcemia and Hypocalcemia In: DiBartola SP, ed. Fluid, electrolyte and acid-base disorders in small animal practice. 3rd ed. St. Louis, Mo;London: Saunders Elsevier, 2006; 122-194. 2. Schenck PA, Chew DJ, Brooks CL. Fractionation of canine serum calcium, using a micropartition system. Am J Vet Res 1996; 57: 268-271. 3. Schenck PA, Chew DJ, Behrend, EN. Updates on hypercalcemic disorders In: August JR, ed. Consultations in feline internal medicine. 5th ed. St. Louis: Saunders, 2006; 157-168. 4. Rosol TJ, Nagode LA, Chew DJ, et al. Disorders of calcium. In: DiBartola SP, ed. Fluid Therapy in Small Animal Practice. 2nd ed. Philadelphia: W.B. Saunders Co, 2000; 108-162. 5. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010; 74: 209-213. 6. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. Am J Vet Res 2005; 66: 1330-1336. 7. Schenck PA, Chew DJ, Brooks CL. Effects of storage on serum ionized calcium and pH values in clinically normal dogs. Am J Vet Res 1995; 56: 304-307. 8. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991-1997). J Vet Intern Med 2000; 14: 184-189. 9. Chew DJ, Schenck PA. Idiopathic hypercalcemia - what do I do?, in Proceedings. The North American Veterinary Conference, 2007; 732-734. 10. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973-1984). J Am Vet Med Assoc 1987;190: 1196-1202. 11. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108-116. 12. Schenck PA, Chew DJ. Determination of calcium fractionation in dogs with chronic renal failure. Am J Vet Res 2003; 64: 1181-1184. 13. Hardy B. Alendronate treatment of idiopathic hypercalcemia in cats (unpublished observations). The Ohio State University, 2008. 14. Messinger JS, Windham WR, Ward CR. Ionized hypercalcemia in dogs: a retrospective study of 109 cases (1998-2003). J Vet Intern Med 2009; 23:514-519. 15. Thompson AL, Scott-Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid-deficient hypoadrenocorticism in dogs: 46 cases (1985-2005). J Am Vet Med Assoc 2007; 230: 1190-1194. 16. Feldman EC, Hoar B, Pollard R, et al. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987-2004). J Am Vet Med Assoc 2005; 227: 756-761. 17. Ham K, Greenfield CL, Barger A, et al. Validation of a rapid parathyroid hormone assay and intraoperative measurement of parathyroid hormone in dogs with benign naturally occurring primary hyperparathyroidism. Vet Surg 2009; 38: 122-132. 18. Wisner ER, Penninck D, Biller DS, et al. High-resolution parathyroid sonography. Vet Radiol Ultra 1997; 38: 462-466. 19. Feldman EC, Nelson RW. Hypercalcemia and primary hyperparathyroidism. In: Feldman ECaN, RW, ed. Canine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis, MO: WB Saunders, 2004; 660-715. 20. Modified from Schenck PA, Chew DJ. Hypercalcemia: a quick reference. Vet Clin North Am Small Anim Pract 2008; 38: 449-453. 21. Adapted from DiBartola SP. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed, St Louis, 2006, Saunders. This article was previously pubished in 2011. |
|
|
vetgrad.com is committed to handling personal data responsibly. Please view our Data Protection Policy |


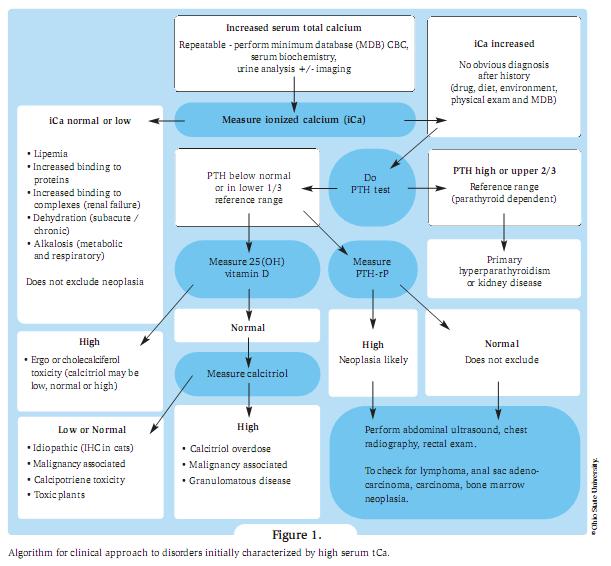
 The likely cause may be obvious from findings in the history or from physical examination, but if the cause is not immediately apparent, imaging with chest and abdominal radiographs and abdominal ultrasound, plus a parathyroid panel (PTH, PTHrp, 25-Vit D) is recommended.
The likely cause may be obvious from findings in the history or from physical examination, but if the cause is not immediately apparent, imaging with chest and abdominal radiographs and abdominal ultrasound, plus a parathyroid panel (PTH, PTHrp, 25-Vit D) is recommended.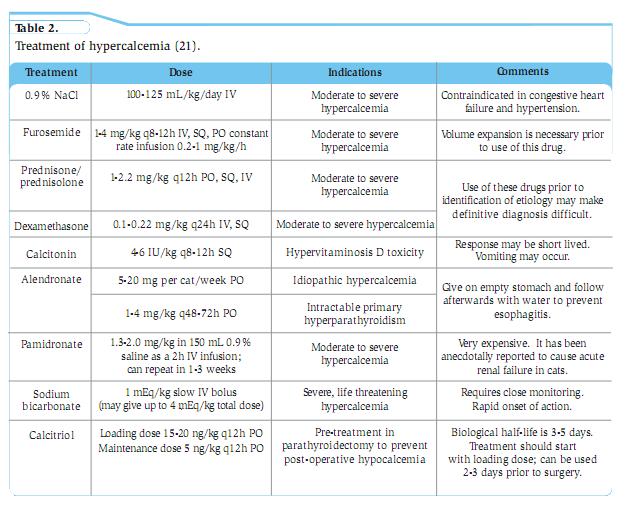 Removal of the underlying cause is the definitive treatment but this is not always immediately possible. The goal of supportive treatment is to enhance urinary excretion of calcium and to prevent calcium resorption from bone. The first tier of treatment is to use parenteral fluid therapy to correct dehydration (as haemoconcentration contributes to increased iCa) followed by furosemide for persistent and severe hypercalcaemia.
Removal of the underlying cause is the definitive treatment but this is not always immediately possible. The goal of supportive treatment is to enhance urinary excretion of calcium and to prevent calcium resorption from bone. The first tier of treatment is to use parenteral fluid therapy to correct dehydration (as haemoconcentration contributes to increased iCa) followed by furosemide for persistent and severe hypercalcaemia.
